
Efforts towards Rh(II)-catalyzed N-alkoxyazomethine ylide generation: Disparate reactivities of O-tethered α-diazo keto and -β-ketoester oximes - ScienceDirect

Efforts towards Rh(II)-catalyzed N-alkoxyazomethine ylide generation: Disparate reactivities of O-tethered α-diazo keto and -β-ketoester oximes - ScienceDirect
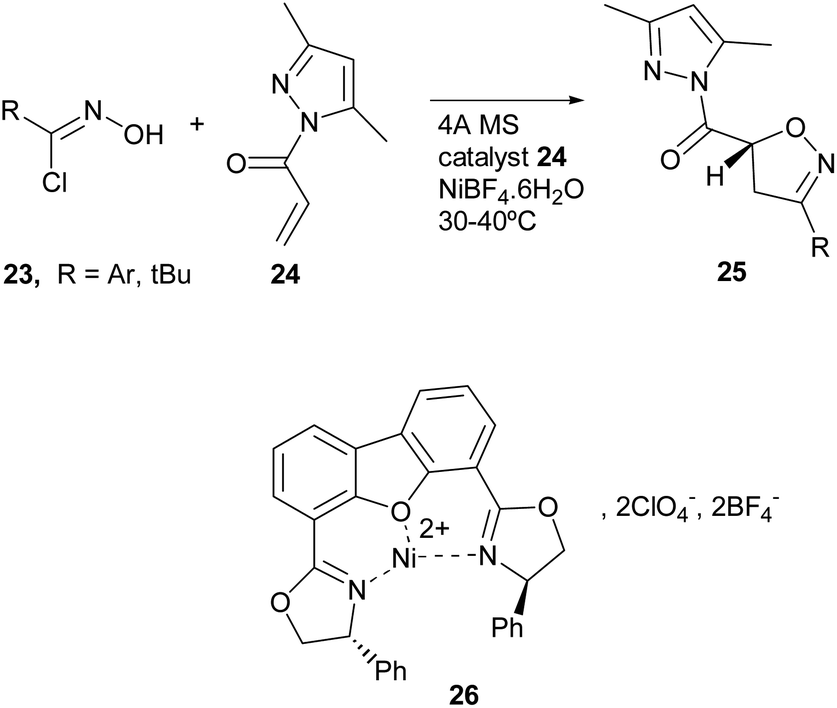
Metal-catalyzed 1,3-dipolar cycloaddition reactions of nitrile oxides - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB02072H
Reverse transcriptase genes are highly abundant and transcriptionally active in marine plankton assemblages

Efforts towards Rh(II)-catalyzed N-alkoxyazomethine ylide generation: Disparate reactivities of O-tethered α-diazo keto and -β-ketoester oximes - ScienceDirect

Efforts towards Rh(II)-catalyzed N-alkoxyazomethine ylide generation: Disparate reactivities of O-tethered α-diazo keto and -β-ketoester oximes - ScienceDirect

Metal-catalyzed 1,3-dipolar cycloaddition reactions of nitrile oxides - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB02072H

Efforts towards Rh(II)-catalyzed N-alkoxyazomethine ylide generation: Disparate reactivities of O-tethered α-diazo keto and -β-ketoester oximes - ScienceDirect
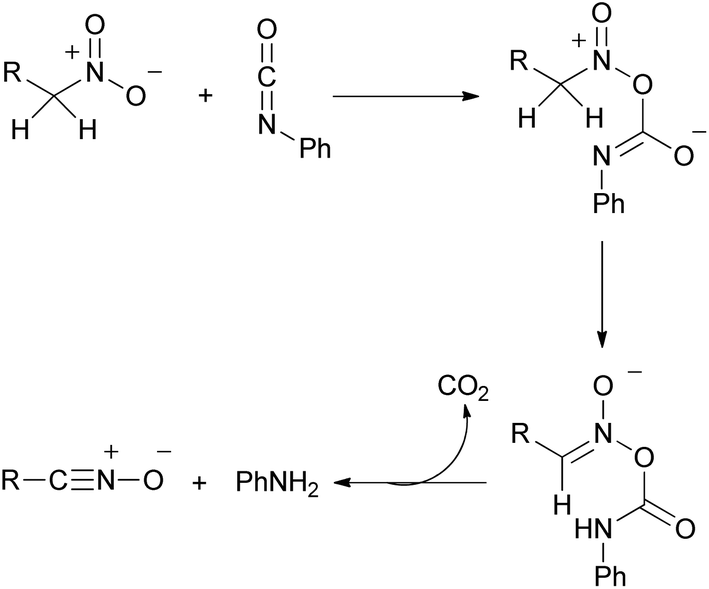
Metal-catalyzed 1,3-dipolar cycloaddition reactions of nitrile oxides - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB02072H









