The dependence of Fe3+ and Fe2+ ions concentration in the solution from... | Download Scientific Diagram

Accelerated Fe3+/Fe2+ cycle using atomic H* on Pd/Al2O3: A novel mechanism for an electrochemical system with particle electrode for iron sludge reduction in the Fe2+/peroxydisulfate oxidation process - ScienceDirect

Roles of Fe2+, Fe3+, and Cr3+ surface sites in the oxidation of NO on the (Fe,Cr)3O4(1 1 1) surface termination of an α-(Fe,Cr)2O3(0 0 0 1) mixed oxide - ScienceDirect
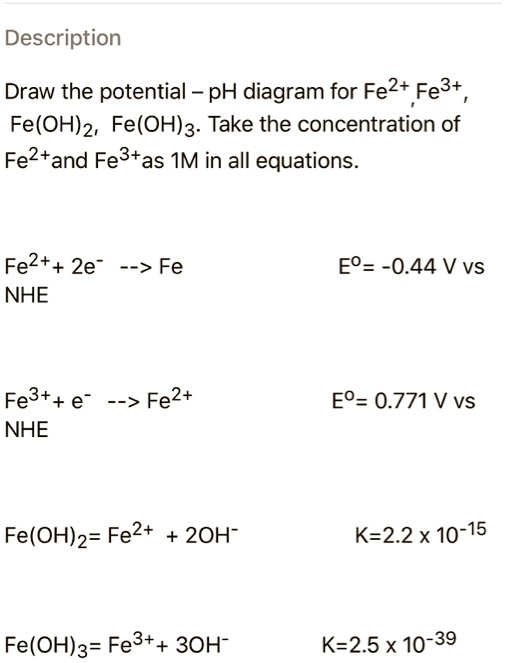
SOLVED: Description Draw the potential - pH diagram for Fe2+ Fe3+ Fe(OHJz, Fe(OH)g. Take the concentration of Fe2+and Fe3+as IM in all equations. Fe2++ 2e –> Fe NHE EO= -0.44 V vs
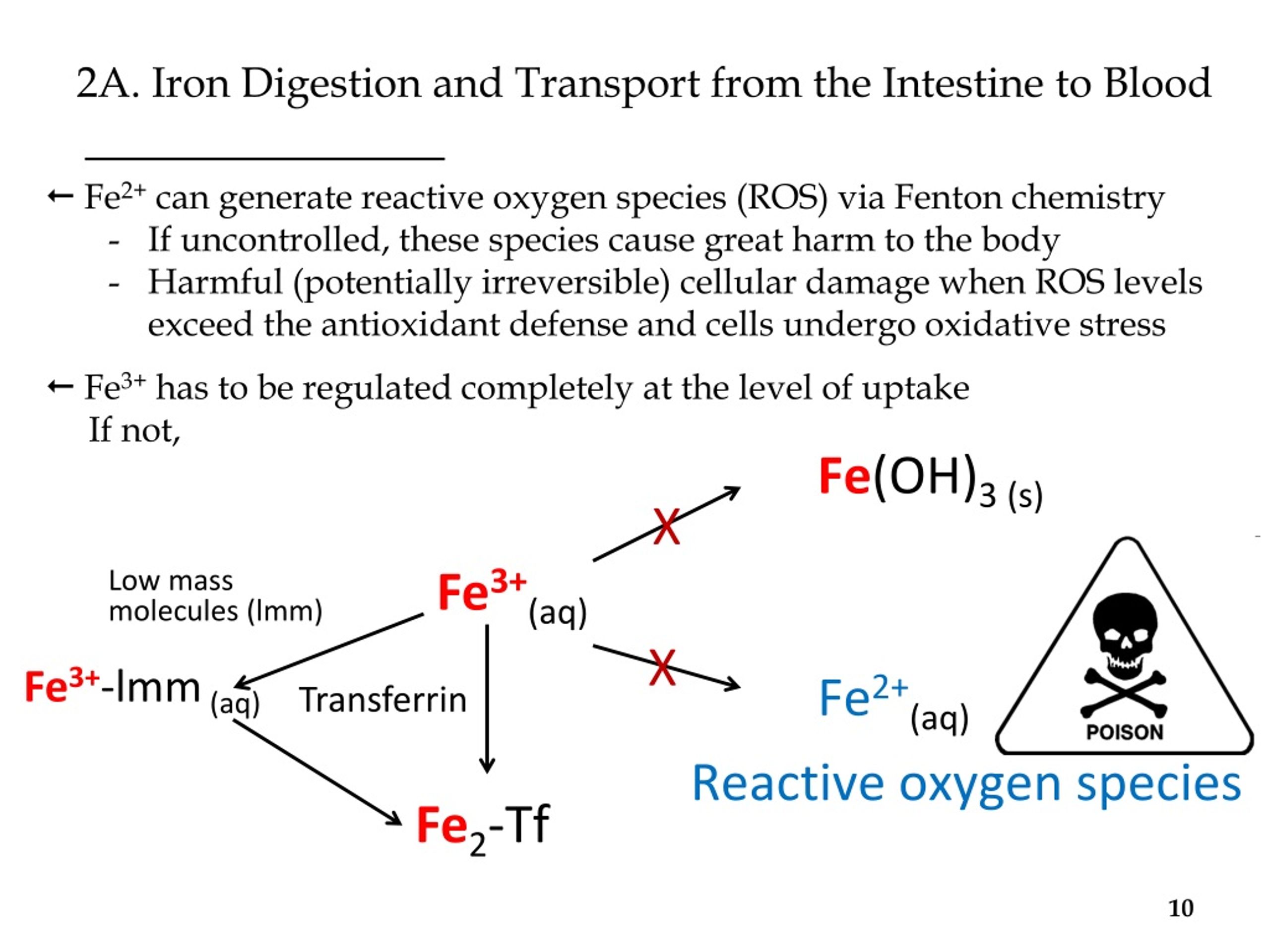
PPT - Iron (Fe 2+ /Fe 3+ ) Transport and Trafficking in Mammals Bertini et al Ch. 5 and 8 PowerPoint Presentation - ID:432903

Fe2+/Fe3+ Cycling for Coupling Self‐Powered Hydrogen Evolution and Preparation of Electrode Catalysts - Chen - 2022 - Angewandte Chemie International Edition - Wiley Online Library

SOLVED: Which statement is not true for the reaction: Fe2+ 5 Fe3+ Fe3+ could be referred to as an oxidizing agent in this reaction Both Fe3+ and Fe2t are called cations Fe3+

Color online) Energetic positions of Fe (Fe1, Fe2, and Fe3) and B (B... | Download Scientific Diagram

Given, standard electrode potentials, Fe^2 + + 2e^ - → Fe, E^o = - 0.440 V Fe^3 + + 3e^ - → Fe, E^o = - 0.036 VThe standard electrode potential (E^o) for Fe^3 + + e^ - → Fe^2 + is:



.PNG)